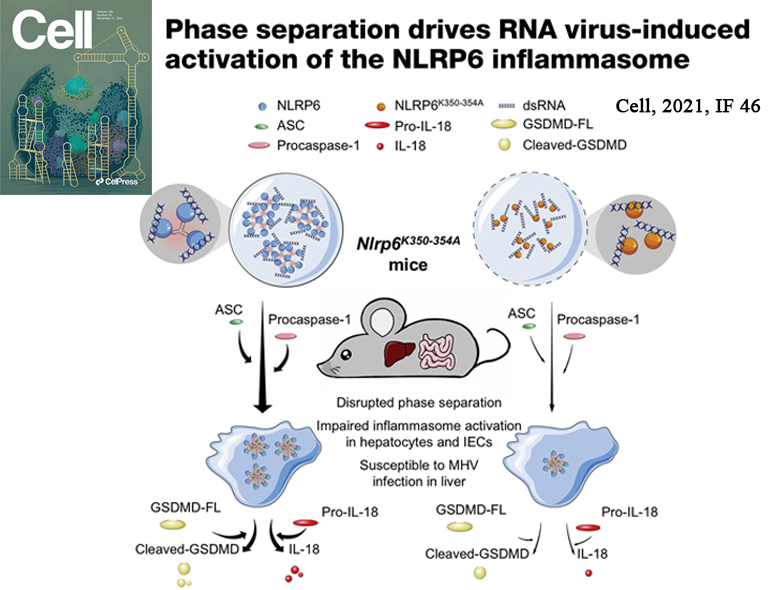
NLRP6 is important in host defense by inducing functional outcomes including inflammasome activation and interferon production. Here, we show that NLRP6 undergoes liquid-liquid phase separation (LLPS) upon interaction with double-stranded RNA (dsRNA) in vitro and in cells, and an intrinsically disordered poly-lysine sequence (K350-354) of NLRP6 is important for multivalent interactions, phase separation, and inflammasome activation. Nlrp6-deficient or Nlrp6K350-354A mutant mice show reduced inflammasome activation upon mouse hepatitis virus or rotavirus infection, and in steady state stimulated by intestinal microbiota, implicating NLRP6 LLPS in anti-microbial immunity. Recruitment of ASC via helical assembly solidifies NLRP6 condensates, and ASC further recruits and activates caspase-1. Lipoteichoic acid, a known NLRP6 ligand, also promotes NLRP6 LLPS, and DHX15, a helicase in NLRP6-induced interferon signaling, co-forms condensates with NLRP6 and dsRNA. Thus, LLPS of NLRP6 is a common response to ligand stimulation, which serves to direct NLRP6 to distinct functional outcomes depending on the cellular context.