The intestinal epithelium maintains homeostasis through sophisticated immune surveillance. NLRP6, a pattern recognition receptor highly expressed in gut epithelial cells, is crucial for host defense, yet its activation mechanism has remained unclear.
On January 6, 2026, our team published a study in *Cell Host & Microbe* titled UBE2O-mediated monoubiquitination licenses NLRP6 inflammasome activation in the intestine. We reveal that the E3 ligase UBE2O catalyzes monoubiquitination of NLRP6 at two specific sites: K680-687 in the LRR domain and K115-130 in the PYD domain. This dual modification is essential for inflammasome activation.
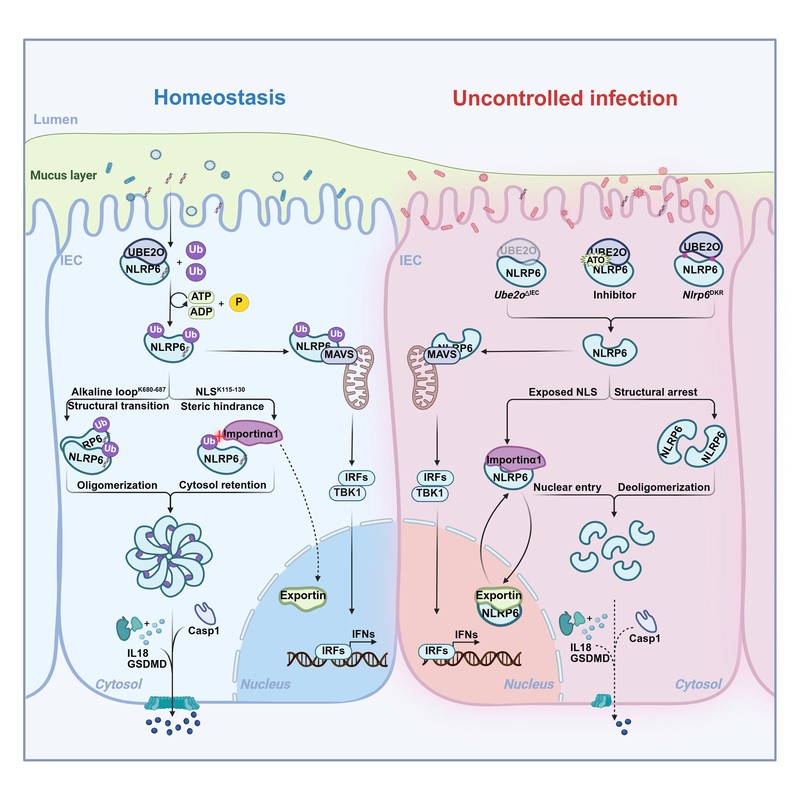
Functionally, UBE2O specifically activates the NLRP6 inflammasome pathway without affecting type I interferon signaling. *In vivo*, intestinal epithelial-specific *Ube2o* knockout mice (*Ube2o*ΔIEC) and mice harboring NLRP6 ubiquitination-site mutations (*Nlrp6*DKR) exhibit defective inflammasome function, including impaired Caspase-1 activation, reduced GSDMD cleavage, and decreased IL-18 secretion.
Clinically, arsenic trioxide (ATO), a UBE2O inhibitor used to treat acute promyelocytic leukemia (APL), suppressed intestinal NLRP6 activation and IL-18 levels in mice. APL patients receiving ATO also showed reduced IL-18, suggesting a mechanism for its gastrointestinal side effects.
Mechanistically, monoubiquitination at K680-687 promotes NLRP6 oligomerization via conformational change, while modification at K115-130 creates spatial hindrance to block nuclear import, ensuring cytoplasmic retention. This coordinated Dual-Safeguard Model enables precise inflammasome assembly.
In infection models, this UBE2O-NLRP6 axis was critical for defense against rotavirus and *Citrobacter rodentium*, as deficient mice showed impaired pathogen clearance.
In summary, we identify UBE2O as a key post-translational regulator of NLRP6 and propose a dual-site monoubiquitination mechanism that ensures its specific activation, advancing our understanding of intestinal immunity and offering potential therapeutic insights.
The Defining Touch: Much like the final brushstrokes that bring a painted dragon to life, UBE2O's two minimal ubiquitin modifications cooperatively transform dormant NLRP6 into its active form to combat pathogens.

This work was led by Professor Shu Zhu (USTC), with postdoctoral fellow Decai Wang and doctoral student Xuequn Chen as co-first authors. It was supported by the National Key R&D Program and the National Natural Science Foundation of China.


